CANKADO’s Companion-APP Solution provides Drug-Optimised Digital Applications for Pharmaceutical Companies
The tool is a drug tailored extension of CANKADO’s e-Health solution
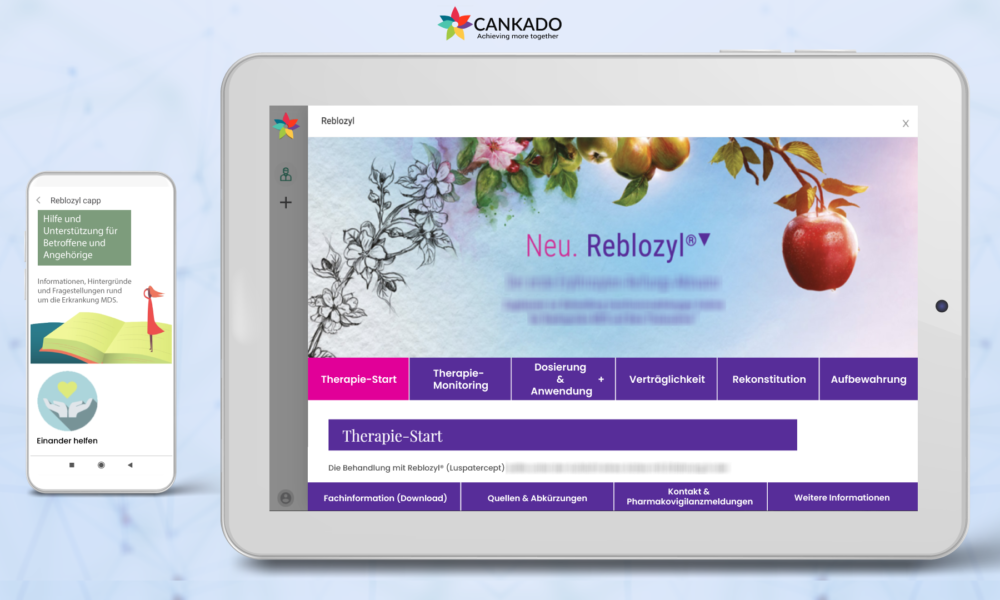
Cologne, Germany, 17.12.2020 – CANKADO’s Companion-APP solution provides an environment that helps to build a drug-tailored digital connected care solution for physicians and their patients. Pharmaceutical companies can easily develop their own multi-dimensional drug-optimized digital support for every drug and treatment. It improves patient safety, increases patient compliance, and prolongs drug adherence for a better outcome.
CANKADO Companion-APP is a complete solution comprising a family of medical devices according to EU and US regulations. It is built on CANKADO’s backbone structure that consists of patient’s records ensuring data security and privacy. It allows connection to healthcare professionals and includes the AI-driven PRO-React framework. Information extensions are integrated on Companion-APP, tailored for each drug/treatment. Pharma partners of CANKADO can build their own drug tailored content management system (CMS) using leading-edge technologies. The CANKADO Companion-APP solution provides a simple, fast, and high-value path for joint development. Through the integral development of a large bundle of Companion-APPs, synergistic effects will improve time to market and reduce development and certification costs.
Celgene GmbH, a subsidiary of Bristol Myers Squibb, has signed a contract with CANKADO to accompany all their drugs in hematology for the German market. The first supported drugs will be Revlimid, Luspatercept and Imnovid.
Companion-App supports physicians and patients effectively during the treatment phase. CANKADO’s Companion-APP is developed based on extensive knowledge and evidence. CANKADO is a constantly evolving system with a continuous development plan.
About CANKADO
CANKADO is a digital health platform with a web/app multilingual system. It is registered as a medical device in the European Union and compliant with the FDA classification for Mobile Medical Devices. CANKADO offers digital health solutions and develops new tools specific to the requirements and outputs of each project. It runs with a wide range of clientele that mostly covers pharmaceutical companies and Clinical Research Organisations (CROs). Click here to enquire further information regarding CANKADO’s digital health services.
COMPANY DESCRIPTION
CANKADO Service GmbH, headquartered in Germany, operates with over 50 employees with 6 offices in four countries and project experience in all continents. CANKADO provides the worldwide leading system for multi-lingual support of patients with chronic diseases in routine care and in clinical trials, also offering various digital health solutions in the areas of cancer, diabetes, cardiology, surgery, cell therapy, psychology, and nutrition.
For more information, please contact
Souja Mol
Head Global Business Development, CANKADO
Phone: +49 221 429 153 04
Email: s.mol@cankado.com
CANKADO ePRO is trusted by
Want to know more?
Contact us to know more about our products and we’ll answer all your queries.
Solutions
Contact Us
- Call: 0800 - 0009212
- (free for calls from Germany)
- Call: 0049 221 429 153 00
- (for international calls)
- Email: support@cankado.com
