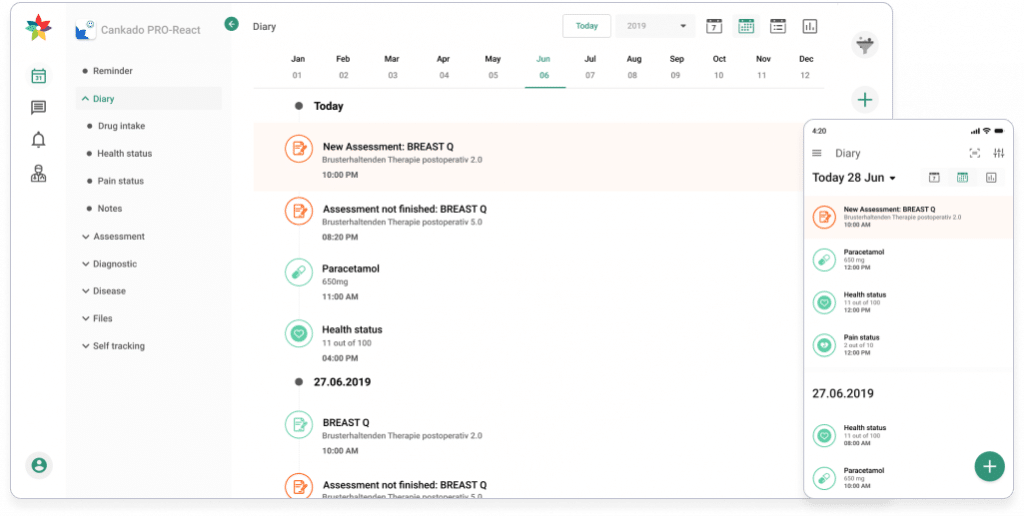
Improving medical care with Cankado
CANKADO provides the environment to build drug-tailored, digital treatment support solutions, called referred to as Companion-APP’s. This environment allows physicians to support all patients under any treatment within one system.